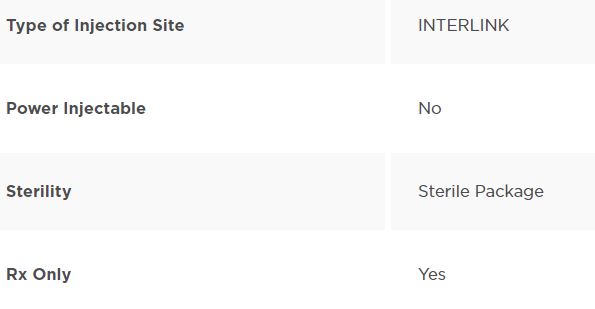
INJECTION SITE LL INTERLINK, 800/CS
SKU
MPSRH-BAX2N3379
In stock
Quick Overview
BAXTER
| Brand | RHS |
|---|---|
| MPN | BAX2N3379 |
| Packaging | CS/800 |
$319.00

Note : Image shown for reference purposes only. Actual product appearance may vary. Please read product description for full and accurate details
Product Description
Baxter 2N3379 - INJECTION SITE LL INTRLNK, 800/CS
INTERLINK Injection Site, Male Luer Lock Adapter. 800 units/ case
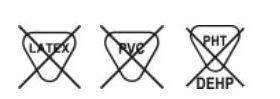
INTERLINK Injection Site, Male Luer Lock Adapter. Non-DEHP. Non-PVC. Sterile Package. Unit Carton of 800.
Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | Yes |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |
Sterilization
| Device Packaged as Sterile: | Yes |
| Requires Sterilization Prior to Use: | No |
DEHP Free
Our IV sets are DEHP free. Infusion Therapy Standards of Practice advise to usee administration sets free of di-ethylhexyl-phthalate (DEHP) to administer lipid-based infusates, such as IVFE or TNA. DEHP is lipophilic and is extracted into the lipid solution with commonly used polyvinyl chloride administration sets and containers. DEHP is considered a toxin, and studies have demonstrated increased DEHP levels in lipid solutions, which is especially a risk with neonatal, pediatric, and long-term home care patients.
Product Characteristics
Packing Information

Write Your Own Review
